ONE DAY IN A LAB

Juliette: Hi, Umi, do you want to go to lunch? We’d better go before the cafeteria gets packed with people.
Umi: Can you wait a bit? I sort of want to finish this before.
J.: What are you doing anyhow?
U.: Well, it is a bit hard to explain, right now anyhow.
J.: I know you work with silver or some such thing, for analysis.
U.: Yah, I do work with silver nanoparticles and a laser, for analysis of molecules.
AN HOUR LATER IN THE SCHOOL CAFETERIA
J.: (Finally finding places to sit and eat) That was a long wait, really. We should have gotten here much earlier.
U.: Sorry about that. I could not stop in the middle of my experiment, but thanks to your patience, I did manage to get a signal from an enzymatic product.
J.: Enzymatic product? What’s the big deal about that?
BACK IN THE LAB
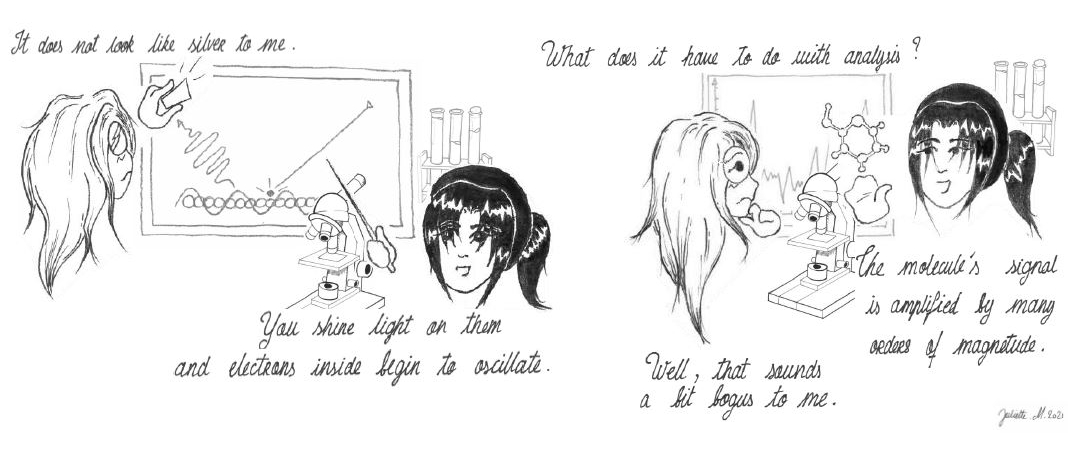
J.: (Looking at a SERS substrate) It does not look like silver to me.
U.: Yes, you are right. It is because of the small size of these silver nanoparticles. At this scale, its light property becomes quite different from usual. You shine light on them and electrons inside begin to oscillate. But because their movement is constrained and they end up generating what is called a near-field, basically a very strong electric field.
J.: What does it have to do with analysis?
U.: If we want to analyze molecules, more specifically find out what they are, then you just place them in the near-field. Then, voila, the signal from them become amplified by many orders of magnitude.
J.: Really? That sounds a bit bogus to me.